Tombstones, although they are associated with death, they are also immortalized symbols aimed at capturing one’s life. With this comes the ability to customize a tombstone to any size, color, and material, but not all tombstones can withstand the elements. Acid rain is water precipitate containing pollutant byproducts that are both acidic and electrolytic in nature. Due to this, acid rain can put a finite stopwatch on a tombstone. So, what types of tombstones can endure the effects of acid rain?
Marble and limestone, or calcium carbonate, is an aesthetically appealing material, but is very susceptible to weathering due to acid rain. Calcium carbonate (CaCO3) is a naturally occurring mineral that has multiple different crystalline structures and acts as a buffer in terms of acid/base chemistry. It serves as a basic salt and can effectively buffer as a carbonate and bicarbonate ion 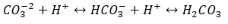 . Acid rain consists of mainly nitric (HNO3) and sulfuric acid (H2SO4) and will react with the basic salt to form calcium sulfate as well as carbonic acid. In this case, we’ll deal with mostly sulfuric acid. The calcium sulfate is soluble in water with a Ksp of 4.93×10-5 and the carbonic acid dissociates into carbon dioxide gas and water (6).
. Acid rain consists of mainly nitric (HNO3) and sulfuric acid (H2SO4) and will react with the basic salt to form calcium sulfate as well as carbonic acid. In this case, we’ll deal with mostly sulfuric acid. The calcium sulfate is soluble in water with a Ksp of 4.93×10-5 and the carbonic acid dissociates into carbon dioxide gas and water (6). 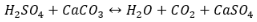 The reaction shown on the left would normally reach equilibrium if it were done in a lab but the reactants are in excess as the gravestone contains a large amount of solid (11). This means that the forward reaction is favored which results in the corrosion of the solid. To further expedite this process, the water is continually being washed away and eliminating any products that would be forming. This also favors the forward corrosion reaction.
The reaction shown on the left would normally reach equilibrium if it were done in a lab but the reactants are in excess as the gravestone contains a large amount of solid (11). This means that the forward reaction is favored which results in the corrosion of the solid. To further expedite this process, the water is continually being washed away and eliminating any products that would be forming. This also favors the forward corrosion reaction.
So, as the acid rain reacts with the marble or limestone, it will slowly corrode the minerals, easily damaging them as seen in the photo below (7).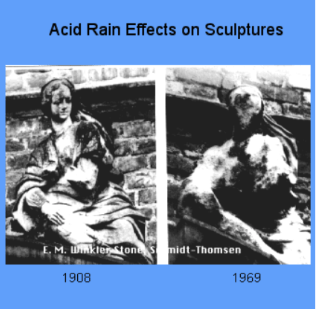
Bronze and white bronze are two metals that are more durable and resistant to weathering, but are still susceptible to acid rain. Bronze and white bronze are both alloys consisting of copper and tin for bronze or zinc, copper, and tin for white bronze. Since copper and tin spontaneously form oxide layers, a layer of copper oxide (and also tin oxide) forms on the outside of the bronze. This layer is known as the patina layer and acts as a shielding mechanism for the metal. The patina surface is formed through basic redox chemistry, occurring mainly between copper metal and the atmospheric oxygen gas. Four moles of copper will oxidize to the “plus one state” as one mole of diatomic oxygen will reduce to the “minus two state” and react to form two moles of copper oxide 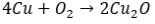 . When it rains, the electrolytic particles in the rain continuously come in contact and interact with the patina to form an electrolyte layer on the outside patina surface. Through these two surfaces’ interactions, corrosion will result at the patina and eventually penetrate the bronze itself. (8). The sulphuric acid in the electrolyte layer will undergo an acid/base reaction with the basic metal oxide patina to form copper hydroxide and copper sulfate
. When it rains, the electrolytic particles in the rain continuously come in contact and interact with the patina to form an electrolyte layer on the outside patina surface. Through these two surfaces’ interactions, corrosion will result at the patina and eventually penetrate the bronze itself. (8). The sulphuric acid in the electrolyte layer will undergo an acid/base reaction with the basic metal oxide patina to form copper hydroxide and copper sulfate 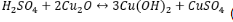 (9). Normally, the patina layer protects against significant corrosion, but when it is composed of copper sulfate, it leads to greater degradation of the tombstone. To measure this, spectroscopy and a colorimetric analysis can be used to show the decrease in luster as corrosion occurs. The metal will lose its shiny hue and change to a greenish-white color over this duration. To prevent bronze tombstones from corroding as fast, it has been found that higher percentages of tin in bronze alloys results in resisting acid rain (8).
(9). Normally, the patina layer protects against significant corrosion, but when it is composed of copper sulfate, it leads to greater degradation of the tombstone. To measure this, spectroscopy and a colorimetric analysis can be used to show the decrease in luster as corrosion occurs. The metal will lose its shiny hue and change to a greenish-white color over this duration. To prevent bronze tombstones from corroding as fast, it has been found that higher percentages of tin in bronze alloys results in resisting acid rain (8).
In order to truly resist the effects of acid rain and weathering on a tombstone, most people will use granite due to its very high durability and resistance to various environmental factors. Granite consists of a high silica content as opposed to marble, limestone, and bronze, which do not. Limestone and marble are both basic salts and bronze forms a basic metal oxide layer, but the silica in granite is primarily a nonmetal oxide. Nonmetal oxides are acidic in nature, so when granite is exposed to acid, its impact is much lesser than other tombstone choices 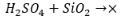 (10).
(10).