In the 1950s, acid rain became measurable, which did not come as a surprise to scientists. They knew that coal plants in the midwest (where acid rain was first observed) were releasing sulfur and nitrogen oxides into the atmosphere, which would reach clouds, and dissolve, making the water acidic 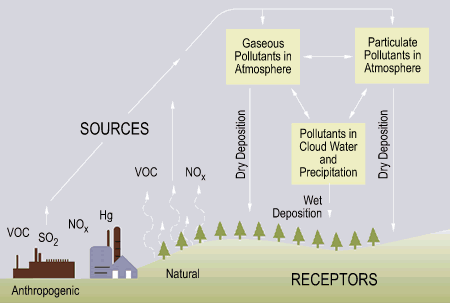 (11). As far back as the 1850s, acid rain had been theorized and categorized but it was the first time that scientists began to study and observe it in nature. Currently, the pH of rain in the United States can be measured as low as 2.4 (figuring regular rain has a pH of around 5.0-5.5 from the carbon dioxide it picks up from the atmosphere) with the average pH of “acid rain” settling in at 4.0. This rain leaches calcium from soil and robs plants of minerals and nutrients. It also has slowly decreased the pH of lakes and rivers, wreaking havoc on these biomes. Not only does it harm fish and microorganisms, but also birds and other animals that depend on aquatic life. Secondary to the massive environmental impacts, human structures are experiencing chemical degradation from the rain. Any and all materials that undergo acid/base and redox reactions with the rain are experiencing increased rates of erosion due to the increasing chemical activity of the rain. This process will be explained in greater depth but the acid in the rain reacts with the base in the tombstone wearing the surface away slowly. This has had a global impact and has taken its toll on many structures, especially gravestones such as the ones depicted here:
(11). As far back as the 1850s, acid rain had been theorized and categorized but it was the first time that scientists began to study and observe it in nature. Currently, the pH of rain in the United States can be measured as low as 2.4 (figuring regular rain has a pH of around 5.0-5.5 from the carbon dioxide it picks up from the atmosphere) with the average pH of “acid rain” settling in at 4.0. This rain leaches calcium from soil and robs plants of minerals and nutrients. It also has slowly decreased the pH of lakes and rivers, wreaking havoc on these biomes. Not only does it harm fish and microorganisms, but also birds and other animals that depend on aquatic life. Secondary to the massive environmental impacts, human structures are experiencing chemical degradation from the rain. Any and all materials that undergo acid/base and redox reactions with the rain are experiencing increased rates of erosion due to the increasing chemical activity of the rain. This process will be explained in greater depth but the acid in the rain reacts with the base in the tombstone wearing the surface away slowly. This has had a global impact and has taken its toll on many structures, especially gravestones such as the ones depicted here:
 (3). Depending on the gravestone material and the particular chemical composition of the rain, tombstones can experience up to 2mm of erosion/century (as measured on an “average” tombstone) (4). This rate might not seem like a lot, but the impacts on older stones is profound as the centuries accumulate.
(3). Depending on the gravestone material and the particular chemical composition of the rain, tombstones can experience up to 2mm of erosion/century (as measured on an “average” tombstone) (4). This rate might not seem like a lot, but the impacts on older stones is profound as the centuries accumulate.